UK Might Roll Out Antibody Tests With at Least 98% Accuracy by the End of the Year
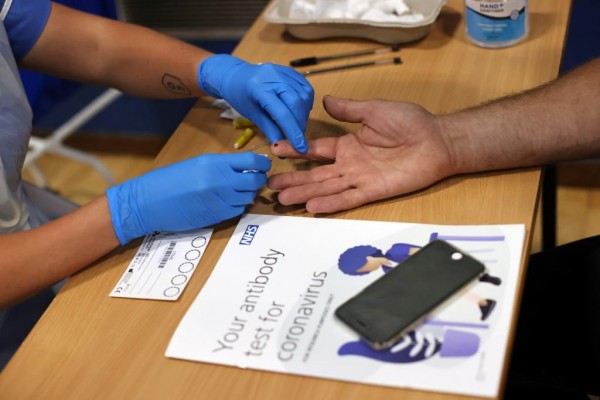
A worker takes a blood sample from a man during a clinical trial of tests for the COVID-19 antibodies, at Keele University, in Keele, Britain.
News reports revealed this week that the United Kingdom might soon roll out millions of antibody tests, which have more than 98 percent accuracy before the year ends.
The antibody tests, which can yield results in just 20 minutes, are supported by the government of the UK last month, have already gone through "secret tests," which have presented that tests have 98.6 percent accuracy.
The trial is developed for home use and is done by finger-pricking, as well as blood-testing for antibodies.
In a report, it was indicated that minsters have already drawn up plans for the distribution of millions of tests across the nation before 2020 ends. The said testing kits, though, the same report indicated, have yet to be verified.
ALSO READ: 5 Simple Ways to Use an At-Home Blood Test Kit
How Finger-Prick Testing Works
Also known as the AbC-19 lateral flow test, the said test has been devised by the UK Rapid Test Consortium--a collaboration between Oxford University and many other diagnostic companies based in the UK.
The test works by blood-drawing through a pricked finger. More so, it works through the use of the full-length spike protein of the virus to identify the so-called IgG antibodies.
The blood is passed through a home strip, showing two pink-colored lines if the test has a positive result. This particular process of testing has resulted in its high sensitivity.
Three batches of thousands of preliminary models have been sent for verification to the UK's Medicines and Healthcare products Regulatory Agency, the information from which is expected in the coming weeks.
Once the test has been approved, the government is looking forward to the kits' online distribution for free.
Indeed, the administration is looking into making the testing products available for free, instead of selling them in supermarkets.
DON'T MISS THIS: Potential COVID-19 Vaccine from University of Oxford Could Activate One's Immune Response
The Possibility of Mass Testing
According to reports, the test initially passed the first major clinical test last month. It was administered to nearly 300 subjects by the Ulster University.
Study results presented that among patients perceived to be positive, the accuracy of the test was 98.8 percent of the time.
Meanwhile, for patients who turned out negative with the result, it was 98.1 percent during that period, producing a total accuracy percentage of 98.6 percent.
The UK-RTC leader and Abingdon Health chairman Dr. Chris Hand said, at this moment, "the test is a tool to collect knowledge."
Hand also said the objective is for the mass testing to be allowed, which you cannot do by sending samples to a laboratory.
By having the ability to test millions, he continued, it will collect information to know the number of people who have antibodies, whether they have immunity from reinfection or their duration of immunity.
The UK administration is hoping to roll out mass testing before the end of 2020. However, it is giving priority to health workers before the general public gets allowed to be tested with it.
Hand explained they are working with the Department of Health when it comes to commercial undertakings, procurement deals, and more.
IN CASE YOU MISSED IT: 20-Minute COVID-19 Blood Test Devised, Australian Researchers Announce
Jul 21, 2020 10:53 AM EDT





