A Brief Guide to Measuring Protein Binding Affinity
Drug discovery is a complex and important part of combating diseases as they arise. The development of new pharmaceuticals and biopharmaceuticals requires researchers to identify the individual molecules that can target, prevent, and treat diseases.
Historically, the process of identifying these molecules took place either by accident-as in the case of the discovery of penicillin-or by repeated testing to isolate which component of a known remedy actively provided therapeutic benefits. Now, researchers use knowledge of how a disease occurs at a molecular level to target specific molecules that may be used to combat it. Studies around the relationship and binding affinity between a potential drug and its target have allowed biopharmaceutical companies to identify therapeutic substances more effectively.
What Is Binding Affinity?
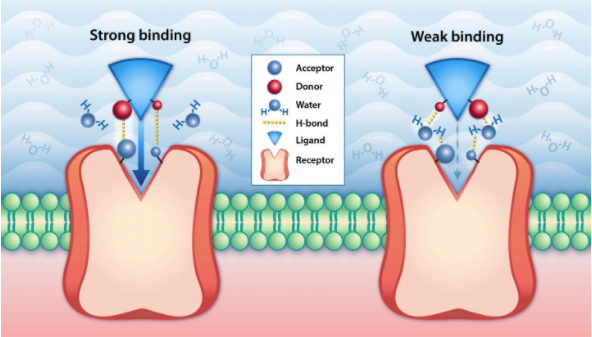
Binding affinity is a crucial part of the drug discovery process. Binding affinity is the relative strength of the interaction between a single molecule and its binding partner, or ligand. Ligands can be a pharmaceutical drug molecule or an inhibitor, designed to halt a particular protein function. The strength of the bond between a molecule and a ligand is affected by the interactions between the two molecules. Common interactions include Van der Waals forces, electrostatic interactions, hydrogen bonding, and hydrophobic interactions.
How Is BInding Affinity Measured?
The affinity of each molecule and its ligand is measured by its equilibrium dissociation constant, or Kd. A smaller Kd value shows a relatively low ability to deviate from a bond and indicates a stronger binding affinity. Similarly, a higher Kd value shows a willingness to separate from a bond and indicates weaker binding affinity.
Researchers use multiple methods to determine the strength of two molecules' binding affinity. Effective ways to measure binding affinity include gel-shift and pull-down assays, equilibrium dialysis, surface plasmon resistance assays, isothermal titration calorimetry, spectroscopic assays, analytical ultracentrifugation, and more. Currently, the industry's gold standard involves the use of MicroScale Thermophoresis technology to determine the number of binding events between the two molecules.
What Role Does Binding Affinity Play in the Drug Discovery Process?
The study of exactly what makes one drug or ligand more effective than another for a particular target is a question that is as old as the process of drug discovery itself. However, while it has long been known that the binding kinetics or affinity between two molecules affect the efficacy of the drug, the understanding of just how this occurs is relatively new. It is known that drugs that bind specifically, quickly, and with stability to their target molecules are much more effective because they are able to remain bound long enough to exert a lasting influence.
However, before the discovery of binding affinity values came along, determining the efficacy of drugs was much less straightforward. Now, binding affinity values can help researchers isolate which drugs have more potential to exert a particular influence on a molecule. In addition, the relative strength of the drug in question can be adjusted once it's clear how long it will remain bound to the target.
With all the recent discoveries around binding affinity, scientists are able to make more informed decisions regarding drug formulations. These decisions continue to influence drug testing and have led to the development of much more stable and effective drugs. As new diseases and remedies await, measuring binding affinity is sure to remain a key component in the drug discovery process.
Sources:
https://new.whatmobile.net/Opinion/article/4-ways-to-use-microscale-thermophoresis
https://www.sciencedirect.com/topics/medicine-and-dentistry/binding-affinity
May 06, 2020 04:57 PM EDT





