Experimental Dengue Vaccine Shows Promise
The world's first dengue vaccine is showing promise in clinical trials, according to an announcement by the vaccination's producer.
According to Sanofi Pasteur, a division of the France-based international drug company Sanofi, the vaccination has shown "satisfactory" and "promising" results in the first of two pivotal Phase III clinical trials designed to determine the vaccination's efficiency and safety.
According to the company, the vaccination achieved its "primary clinical endpoint" for the first of these two trials, by display strong evidence of its efficiency. A total of 10,275 children ranging from two to 14 years old who live in areas that face high dangue infections rates in Indonesia, Malaysia, the Philippines, Thailand, and Vietnam were selected to participate in the study for two years.
The selected children were randomly assigned to receive either three doses of the experimental dangue vaccination or a placebo over the course of a 18 months.
At the end of the study, the children in the vaccine group showed a 56 percent reduction in number of dengue infections, compared to the number of children who became infected in the placebo group.
According to Olivier Charmeil, President and CEO of Sanofi Pasteur, this result reflects the company's aim to develop a vaccination that could directly help the world achieve the WHO's (World Health Organization) ambition to reduce dengue mortality by 50% and morbidity by 25% by 2020."
Of course, if the vaccination proves equally effective and safe in its second Phase III trial -- which is ongoing in Latin America and includes more than 20,000 volunteer subjects -- Sanofi stands to corner a portion of the vaccination market, as no company has yet to produce an approved vaccination for dangue.
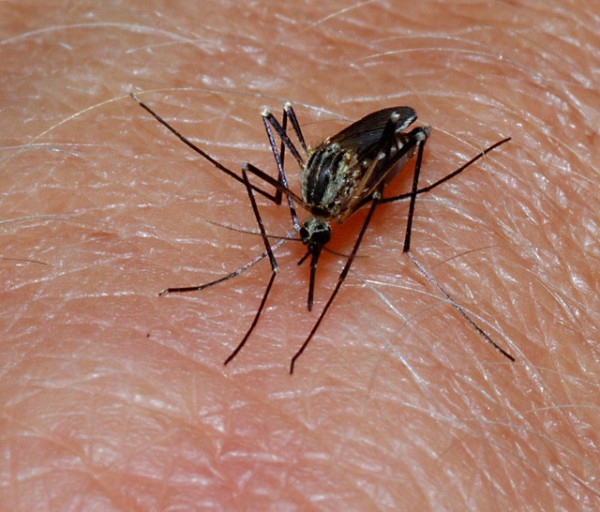
According to the U.S. Centers for Disease Control and Prevention, gangue infections are cause by any one of four viruses transmitted through mosquito bit. While uncommon in the U.S., the infection affect an estimated 400 million people annually and is a leading cause of illness in many tropic and subtopic regions of Asia and Latin America.
The Sanofi Pasteur announcement was first made public on April 28 through a press release.
Details on the Phase III trials can be found here.
Apr 28, 2014 05:36 PM EDT





