Anesthetic Products Recalled for Metal Contaminant
Drug company Hospira Inc. has launched a nationwide recall of two anesthetic injection products that have been identified to contain tiny metal particles that can potentially harm patients.
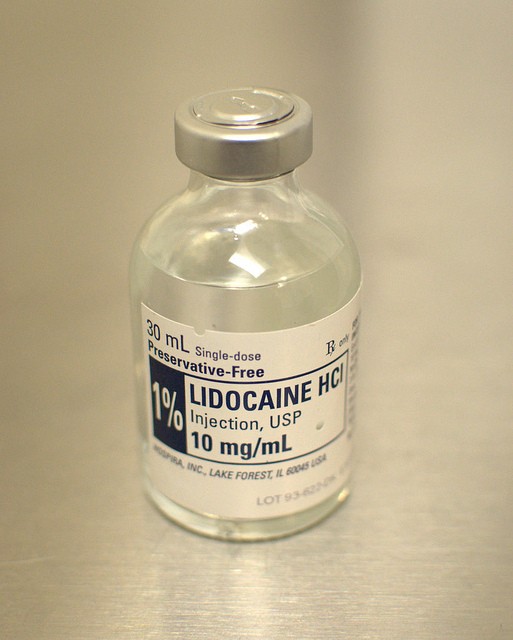
According to the United States Food and Drug Administration (FDA), Hopsira is voluntarily recalling seven lots of Propofal Injectable Emulsion and one lot of their one percent Lidocaine HCI Injection after customers found unusual "visible particulates" lining the neck of the products' bottles and even in the solutions themselves.
A FDA recall announcement made last Thursday revealed that Hospira and the FDA launched recall efforts on April 2 after customers reported free-floating metal particles in the vials of propofal -- an injectable sedative agent used to induce and maintain anesthesia during certain medical procedures.
Last Friday, Hopsira announced a second recall after an emergency investigation looking into production revealed that one lot of lidocaine HCI injections also had been contaminated with a similar metallic particulate, confirming a single customer report that had mentioned free-floating orange and black metallic flakes in the formula's solution. Like propofal, lidocaine is a common local anesthetic that is administered to patients in diluted amounts.
According to the FDA, even being so tiny, these particles can pose a significant threat to patients if they make it into the blood stream via injection. Allergic reactions, inflammation, granuloma formation, and even blockage of the blood pathway may occur if enough of the particulates are in the injected solution.
Both products were distributed nationwide, but the FDA and Hospira have immediately informed hospitals and suppliers to send the contaminated products away for return processing.
No adverse reaction to the aforementioned lots have been reported but medical professionals have been urged to keep special care of which products they are using, especially on patients with a history of cardiovascular trouble.
The propofol release was published on April 17.
The lidocaine release was published on April 18.
Apr 21, 2014 04:22 PM EDT




